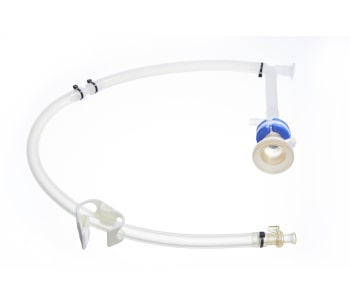
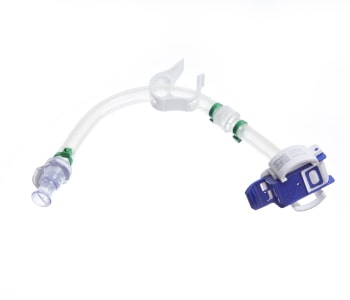
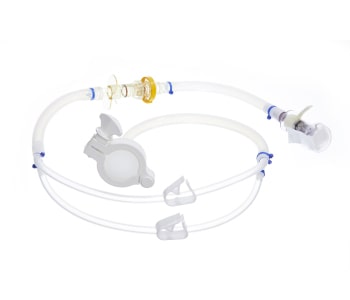
The GMPTEC tubing assemblies are available sterile and ready-to-use or as non-sterile tubing systems. We will provide you with the best material depending on your requirements, whether the tubing assembly has to be autoclavable or gamma-sterilizable. GMPTEC always uses tubing which are already specified and validated, in order to simplify the validation of the whole assemblies for GMP processes.
Tubing Assemblies
Description
Individual Single-Use Tubing Assemblies
Our cGMP tubing assemblies are produced and also packaged in an ISO class 7 cleanroom. Even sub-components like tubing, sanitary fittings, sterile connectors and more are quality inspected before these are being brought into the cleanroom. If necessary, there will be an additional cleaning process in order to assure the highest quality.
The single-use tubing assemblies are manufactured according your requirements, independent of which type of connection you chose. We offer every possible solution, starting with the simple and also cost-efficient solution of a cable tie connection, to BarbLocks and Oetiker-clamps up to overmolding connections.
After an evaluation and analysis of your cGMP application, we will assist you in the choice of the best materials and components. Our goal is to create a tubing assembly, which entirely meets the users requirements. It is of highest importance, that there will be no mistakes during the development phase. It´s because the finished product will be delivered sterile and ready-to-use. So, if a mistake is noticed after delivery, it is usually already too late, since an adjustment won’t be possible without contamination.
Based on many years of experience within the industry, you can rely on the expertise of our specialists. The whole engineering of an assembly will be free of charge. That means you to will just make a decision, when the single-use assembly is already developed and ready to be ordered. For the evaluation phase, at GMPTEC there won’t be high costs or larger order quantities for your initial samples.
GMPTEC stands for the highest quality in the market. Because of our cleaning procedures and a 100% 4-eyes inspection of all assemblies, we deliver the cleanest single-use assemblies coming from a class 7 cleanroom.
Besides this, GMPTEC is also known for the fastest engineering and responds times. The biopharmaceutical industry always requires a fast and good support, when it´s about manufacturing equipment and therefore GMPTEC is the best choice when you develop new single-use assemblies!
Tubing Assemblies Applications
- Upstream- and also downstream processes
- Fill and finish
- Transfer of media and solutions
- Tubing manifolds
- Tubing distributions
- Single-use filtration sets
- Connection of containers and bioreactors
- Vent tubing assemblies
- Filling assemblies
- PUPSIT-Assemblies
- Sampling
Tubing Assemblies are compliant to:
- USP Class VI
- cGMP
- USP <88>
- Endotoxin statement
- BST/TSE confirmation
- Sterilizable
- Irradiation up to 40kGy
- Autoclavable
- Clean room ISO class 7 production
- Use of any kind of tubing, filters, connectors, couplers, flanges and many more