GMPTEC Products
GMPTEC Products
GMPTEC
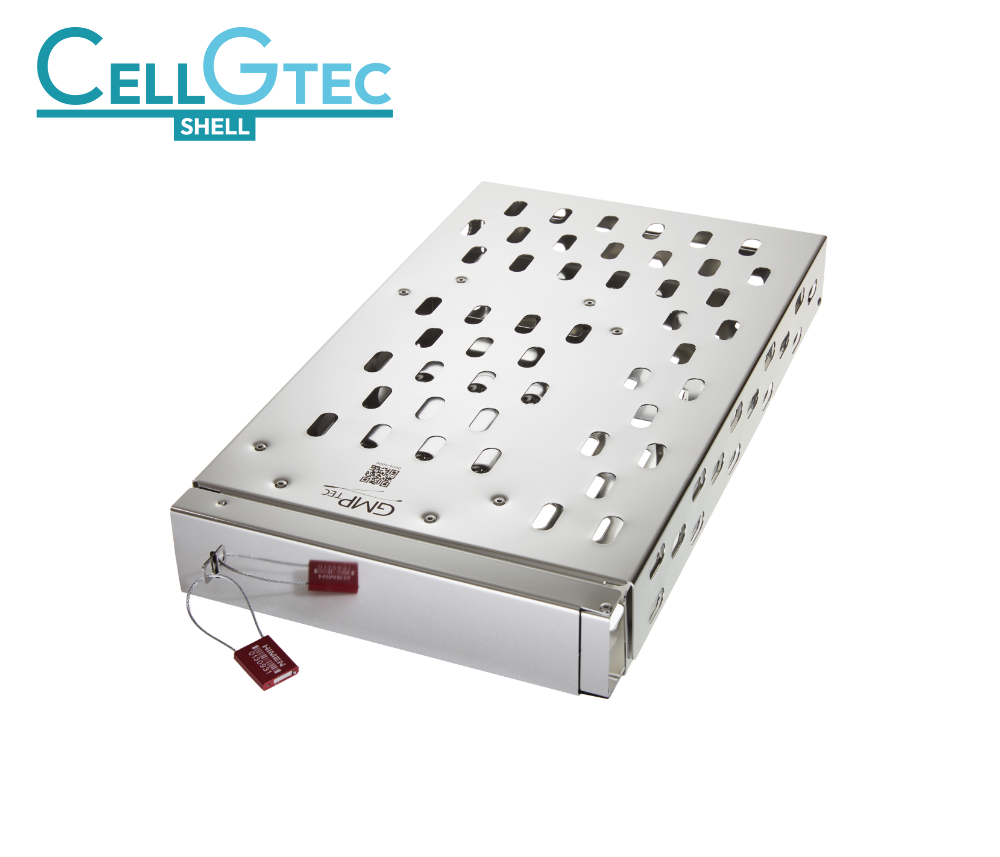
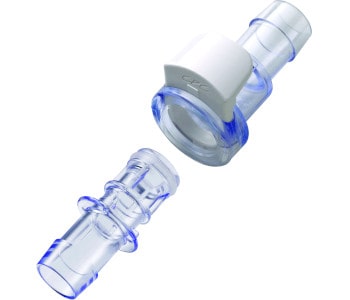
The GMPTEC product portfolio includes a huge variety of components and systems for the production of pharmaceuticals, also diagnostics, chemical products and high-quality foods. Most of the GMPTEC technologies are being used in the biotechnology and both, our single-use and multi-use components, are suitable for cGMP conform biotech-applications. GMPTECs primary goal is to cover all requirements out of one source! This does not only include a huge range of different products, but also services like consulting, assessment and troubleshooting, as well as development and construction.
In terms of customized assemblies, we will gladly take care of the whole product development process! From the first draft, to a technical drawing, to a competitive offer – completely free of charge! Our cleanroom services allow us to create another added value for your processes! We do not only deliver off-the-shelf products in big standardized packaging units: To meet your individual requirements we can clean single GMPTEC goods, channel them into the ISO class 7 cleanroom, also label or laser engrave them, repackage them individually and deliver them directly to you. Right from the start, we make your solutions and interests to our first priority!
We are always available by phone or e-mail and willing to schedule meetings on site even at short notice. The core competence of our team is their wide technical knowledge about the production technologies on the market. We do not settle for only few key products. Instead we want to offer seamless solutions, even if it should be beyond our own portfolio.
GMPTEC
The GMPTEC product portfolio includes a huge variety of components and systems for the production of pharmaceuticals, also diagnostics, chemical products and high-quality foods. Most of the GMPTEC technologies are being used in the biotechnology and both, our single-use and multi-use components, are suitable for cGMP conform biotech-applications. GMPTECs primary goal is to cover all requirements out of one source! This does not only include a huge range of different products, but also services like consulting, assessment and troubleshooting, as well as development and construction.
In terms of customized assemblies, we will gladly take care of the whole product development process! From the first draft, to a technical drawing, to a competitive offer – completely free of charge! Our cleanroom services allow us to create another added value for your processes! We do not only deliver off-the-shelf products in big standardized packaging units: To meet your individual requirements we can clean single GMPTEC goods, channel them into the ISO class 7 cleanroom, also label or laser engrave them, repackage them individually and deliver them directly to you. Right from the start, we make your solutions and interests to our first priority!
We are always available by phone or e-mail and willing to schedule meetings on site even at short notice. The core competence of our team is their wide technical knowledge about the production technologies on the market. We do not settle for only few key products. Instead we want to offer seamless solutions, even if it should be beyond our own portfolio.
NEWS
Positive new construction update: ERDF funding commitment is in!
Christmas party
After the last working day of the year, we at GMPTEC GmbH came together to celebrate our Christmas party and reward the hard work of 2025.
This year has been marked by strong growth, dedication, and true team spirit. Everyone gave their best — and it showed in the great atmosphere during the GMPTEC Winter Games. (more…)
Featured in Local News!
Featured in Local News!
We’re proud to share that several local media outlets reported today about the groundbreaking ceremony for our new GMPTEC GmbH headquarter in Gronau West!
A heartfelt thank you to everyone involved, our amazing team, our partners, and the Gronau community, for making this milestone possible.
Single-Use Event – Leiden
Yesterdays Single-Use Event has been our 4th participation in this show. It has grown incredibly over the last years and we are proud to be a part of it! Thanks to Bas van den Engel and his team for the organization and accomodation. And also special thanks to our BeNeLux Agent Ron van Dooren from Flowmeister B.V. for your support!
Single-Use Event in Leiden 2025
On September 16th, GMPTEC GmbH will once again be present at the Single-Use Event in Leiden at the Corpus Congress Centre. Together with our dutch agent, Flowmeister B.V. (represented by Ron van Dooren), we are excited to showcase our capabilities. As the fastest and most flexible provider of single-use assemblies, we pride ourselves on delivering innovative solutions that truly stand out. We look forward to connecting with partners, customers, and friends in Leiden! #SingleUse #Biopharma #GMPTEC
Our Building Permit Has Been Approved
It’s Official: Our Building Permit Has Been Approved! 🏗️ 🚀
We’re proud to share that GMPTECs next chapter is about to begin and our new headquarters project is moving forward, starting this year.
Here’s a first look at the future:
Located in Gronau West, approx. 800 m² of modern office space across two floors and a 1.600 m² warehouse & production hall, including an ISO Class 7 Cleanroom. (more…)