Information about AseptiQuik W Sterile Connectors
The nominal 1 ½” flow rate of the AseptiQuik W sterile connectors makes it possible to realize significantly higher flow rates and larger transfer volumes than previously possible with alternative sterile connectors. As a result, throughput times can be minimized and productivity significantly increased, even in critical processes.
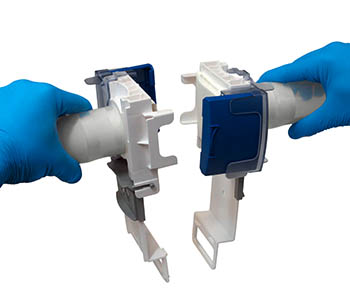
The AseptiQuik W sterile connectors, short AQW, are available with both hose connection and tri-clamp flange. For a complete overview of the available configurations and nominal sizes, please refer to the corresponding tab on this page.
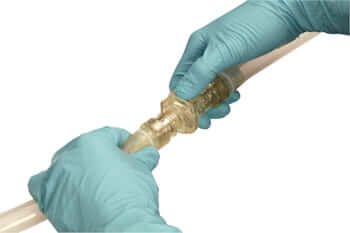
The AseptiQuik W is operated according to a simple principle that ensures reproducible processes and avoids user errors. First, the white pull tab is released from the transport lock and folded down. A click confirms that the end position has been reached. Next, the two coupling halves are aligned opposite each other and pushed into each other. Here, too, a click confirms correct assembly. Now the two white pull tabs of the diaphragm are clicked into each other and then pulled out evenly. This removes the membrane paper and releases the sterile connection. All that remains to be done is to remove the transparent transport locks and close the blue latches.
In this video you can watch the process again in detail:
https://www.youtube.com/watch?v=HKaK_-zJ-5U
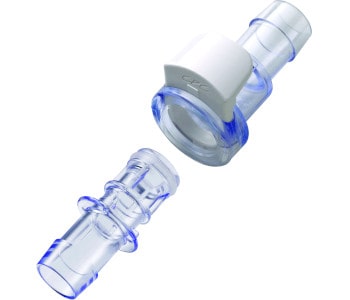
The housing of the AseptiQuik W is made of polycarbonate and the internal seal is made of silicone. All materials meet the highest quality requirements and are suitable for use in biopharmaceutical processes. Extensive validation documentation and extractable data are available on request.
Thanks to the materials used, the AseptiQuik W are suitable for sterilization by autoclave as well as by gamma irradiation. Gamma irradiation can be performed at up to 50 kGy and autoclaving at up to +130 °C for a period of 60 minutes. Thus, the sterile couplings can be used flexibly for different processes and applications.
The AseptiQuik W are ideally suited for single-use systems and can be assembled in our ISO Class 7 clean room as part of customer-specific assemblies. The system is designed according to customer specifications and is perfectly adapted to the individual process parameters. If you are interested, our technical contact persons are at your disposal!
Features and Characteristics
- Genderless
- Robust design
- High flow rates
- Quick and easy handling
- Reduced risk of operator errors
- No additional equipment required
- Gamma stable
- Autoclavable
- ISO class 7 cleanroom production
Processes and Applications
- Single-Use Systems
- Sterile Transfers
- Fluid Handling
Conformitites and Properties
- USP Class VI
- FDA
- No animal derived ingredients (ADCF)
- Validation Guide available
- Extractable Data available