Yes, GMPTEC assemblies can be marked or labeled individually. In general, we offer a variety of different cleanroom services. This includes individual packaging and labeling based on your requirements.
Single-Use Assemblies
Our single-use assemblies are ready-to-use systems for the pharmaceutical industry. Depending on your needs, tubing, bag, bottle, or filter assemblies are available in both ready-to-use and ready-to-autoclave single-use versions.
- GMP-compliant & ready-to-use: All assemblies meet the highest quality standards and can be used directly in production.
- Customized design: Together with you, we develop custom-made single-use systems according to your specifications – efficient, safe, and modular.
- Small order quantities: Thanks to our flexibility, you can also obtain customized assembly solutions for smaller requirements – at attractive conditions.
Our systems are characterized by their versatility: multi-use components are particularly robust and designed for frequent reuse after cleaning and steam sterilization. Single-use components, on the other hand, are usually double- packaged and gamma sterilized, so that they can be safely disposed of after a single use. These systems are generally more cost-effective and do not require autoclaving.
Another advantage: GMPTEC is completely unrestricted and independent in the selection and combination of subcomponents. This enables us to offer solutions that are not only economical but also future-proof and easily integrable into existing processes.
Single-Use Assemblies in the Pharmaceutical Industry
Single-use assemblies in the pharmaceutical industry aren’t just a trend anymore, instead they’ve become an established standard in many large production companies. No matter if CMO/CDMO or companies who produce their own products, single-use technologies are being used nearly everywhere and partly replaced common multi-use systems completely. Especially the biotechnological drug production is a type of production, which uses the same or very identical production steps for several products. Because of this, the choice of single-use technologies for cell culture, upstream- and downstream processes and even filling processes is growing constantly. Upstream bioreactor assemblies are especially complex, because they require the combination of many technologies which need to function within a sterile and closed system. If and how these new technologies will replace traditional bioreactors in the future, will heavily depend upon the further development of the manufacturing process and the single-use technologies related to the vision of “continuous processing”. This describes a current trend and basically means that the production won’t be batch-wise anymore. Proteins and other active substances will be produced and processed continuously, beyond the size of a regular batch. Most likely, single-use continuous production systems will not produce perpetually, but the time until the necessary replacement of disposables will be a lot larger compared to a regular batch-wise production. Nonetheless, it would be conceivable that the used assemblies are going to be replaced in a time-displaced manner, in order to realize the continuous processing.
Setup and Connection Types of Single-Use-Technologies
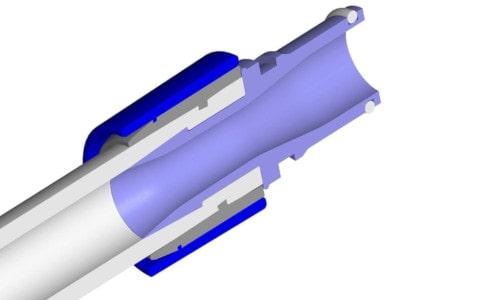
Single-use systems should consist of plastic components only, in order to reduce the costs and allow an easy waste disposal. Components made of stainless steel should be avoided and are rarely used. One example are Oetiker clamps, which are used to fix the tubing (see figure below).
Assembly with Oetiker Clamps for Fixation
These stainless steel clamps cover the complete circumference of the tubing. They are crimped at one or two sides by using a tool and securely fixate the tubing on the hose barb. Assemblies often consist of different sized tubing and hose barbs which need to be connected. Oetiker clamps are a flexible and robust solution and an ideal choice when several different assembly types are being used.
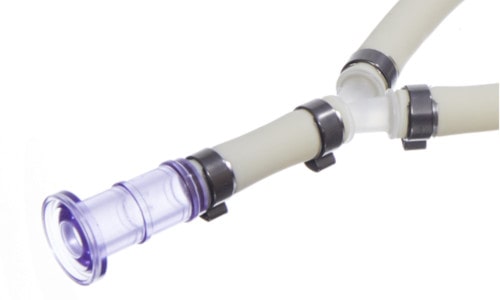
Another type of connection are Barblocks® or PureFit® retainer made by Saint-Gobain (see figure below). Technically this is the only type of connection among the three most popular ones, which has been especially developed for pharmaceutical systems. It consists of plastic collars which are placed on the tubing and crimped together by using a special tool, once the tubing has been pushed over the hose barb. This connection type does not include any steel component and spreads the clamping force the most equal and across the biggest surface area. The choice of the correct sizes is a critical step and can easily lead to the purchase of incorrect parts, which can cause costly delays.
The regular cable tie is still the most used type of connection (see figure below). The advantages are significant, since cable ties will basically fit every time. Within a single-use system, they can be used for nearly every type of tubing of hose barb. Besides that, the regular length of a cable tie will fit most components, regardless their size. The safety factor for using cable ties within single-use assemblies has also been considered. Connections can be secured not by just one, but two cable ties, which could be fitted into opposite directions, in order to avoid leakages. Special tools allow to apply the same amount of torque and automatically cut off the remaining cable tie.
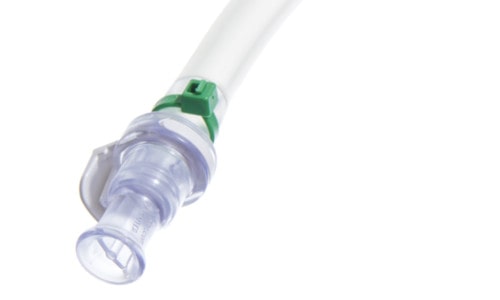
Assembly with Cable Tie for Fixation
Assembly Applications and Assembly Categories
- Single-Use Continuous Processing
- Batch-Wise Single-Use Systems
- Manifold Systems
- Depth Filtration
- Sterile Filtration
- Product Transfer
- Sterile Filling
- Buffer Medium
- Connections Sets
- Mixing Assemblies
- Tubing Sets
- Intermediate Transport and Transfer
- Active Substances Transport and Transfer
- Fermentation
- Sampling Assemblies
- Bio-Reactor Kits
- Single-Use Manifolds
- Pump Assemblies
- Research and Development (low order quantities)
Single-Use Assemblies are compliant to:
- USP Class VI <88>
- BSE/TSE Statement (ADCF)
- FDA CFR 177.1520
- Cleanroom ISO Class 7 or 5 Production
- Gamma Resistant
- SAL 10-6 (Sterility Assurance Level)
- Integrity Testing
- Autoclavable
- REACH
(Certificates and conformities can vary depending on the choice of materials)