GMPTEC Products
GMPTEC Products
GMPTEC
The GMPTEC product portfolio includes a huge variety of components and systems for the production of pharmaceuticals, also diagnostics, chemical products and high-quality foods. Most of the GMPTEC technologies are being used in the biotechnology and both, our single-use and multi-use components, are suitable for cGMP conform biotech-applications. GMPTECs primary goal is to cover all requirements out of one source! This does not only include a huge range of different products, but also services like consulting, assessment and troubleshooting, as well as development and construction.
In terms of customized assemblies, we will gladly take care of the whole product development process! From the first draft, to a technical drawing, to a competitive offer – completely free of charge! Our cleanroom services allow us to create another added value for your processes! We do not only deliver off-the-shelf products in big standardized packaging units: To meet your individual requirements we can clean single GMPTEC goods, channel them into the ISO class 7 cleanroom, also label or laser engrave them, repackage them individually and deliver them directly to you. Right from the start, we make your solutions and interests to our first priority!
We are always available by phone or e-mail and willing to schedule meetings on site even at short notice. The core competence of our team is their wide technical knowledge about the production technologies on the market. We do not settle for only few key products. Instead we want to offer seamless solutions, even if it should be beyond our own portfolio.
GMPTEC
The GMPTEC product portfolio includes a huge variety of components and systems for the production of pharmaceuticals, also diagnostics, chemical products and high-quality foods. Most of the GMPTEC technologies are being used in the biotechnology and both, our single-use and multi-use components, are suitable for cGMP conform biotech-applications. GMPTECs primary goal is to cover all requirements out of one source! This does not only include a huge range of different products, but also services like consulting, assessment and troubleshooting, as well as development and construction.
In terms of customized assemblies, we will gladly take care of the whole product development process! From the first draft, to a technical drawing, to a competitive offer – completely free of charge! Our cleanroom services allow us to create another added value for your processes! We do not only deliver off-the-shelf products in big standardized packaging units: To meet your individual requirements we can clean single GMPTEC goods, channel them into the ISO class 7 cleanroom, also label or laser engrave them, repackage them individually and deliver them directly to you. Right from the start, we make your solutions and interests to our first priority!
We are always available by phone or e-mail and willing to schedule meetings on site even at short notice. The core competence of our team is their wide technical knowledge about the production technologies on the market. We do not settle for only few key products. Instead we want to offer seamless solutions, even if it should be beyond our own portfolio.
NEWS
Exciting News from GMPTEC GmbH!
We are thrilled to announce our new partnership with Neu Industries Pte Ltd as our distributor in Singapore. This collaboration marks an important milestone in our international growth strategy, allowing us to bring our innovative Single-Use Systems and CELLGTEC Freezing Equipment to the Singapore market.
Through this partnership, we are confident in delivering our cutting-edge solutions to a broader audience, supporting efficiency and quality in the biotech and pharmaceutical industries.
We’re proud to be working with Timothy Neui, Paul Neui and their team, who share our commitment to excellence and innovation.
Together, we’re opening doors to new opportunities and driving advancements in the industry.
For inquiries and more information, feel free to reach out to us or connect directly with Timothy Neui at Neu Industries Pte Ltd.
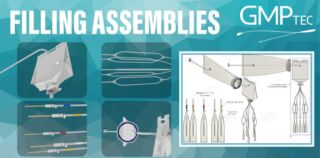
Single-use assemblies for Fill & Finish applications
Excited to share that GMPTEC GmbH has delivered numerous tailored single-use assemblies for Fill & Finish applications in the pharmaceutical industry this year!
Produced in our ISO Class 7 cleanroom, these solutions can include Beta Bags for isolators and single-use filling needles suiting machines of leading manufacturers like Bosch, Optima, and Bausch + Ströbel. What sets us apart? Our unmatched flexibility in designing customized solutions to meet our clients’ unique needs.
Single-Use Event in Leiden 2024
Live from the Single-Use Event in Leiden!
We are having an incredible day at our booth showcasing the CELLGTEC Shell freezing cassette! The response has been fantastic, and it’s great to see so many people interested in our innovative solution. A big thanks to everyone who has stopped by so far! If you’re at the event, come and visit us – we’d love to chat!
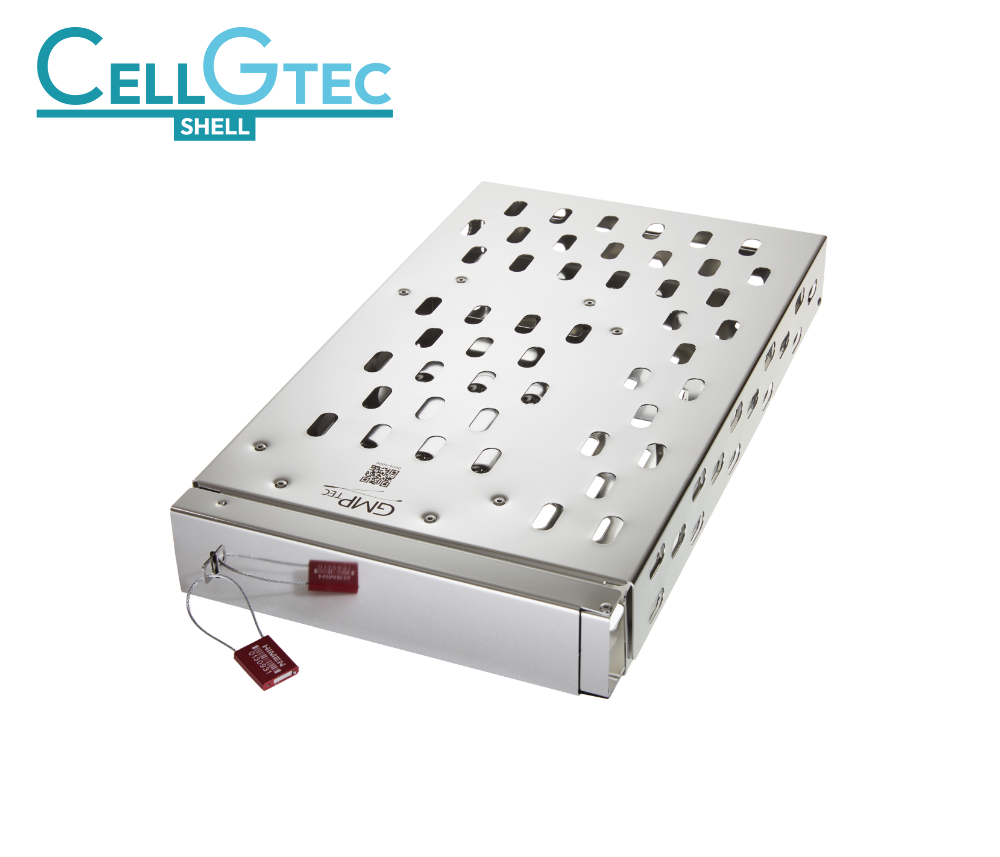
Our Innovation – the CELLGTEC Shell
We are thrilled to introduce our latest innovation, the CELLGTEC Shell, at the Single-Use Event in Leiden tomorrow! 🚀 This groundbreaking freezing cassette is designed to protect polymer bags containing pharmaceutical liquid substances during freezing, storage, and transport at -80°C and lower. Visit us at our booth to learn more about how the CELLGTEC Series enhances product safety in extreme conditions. For more details: CELLGTEC Shell
Annual summer party
🌞 What an amazing weekend! 🌞
Last weekend, we celebrated our annual summer party, and it was a blast! 🎉 We had a fantastic time with great music, refreshing drinks, and lots of fun, all in the company of our wonderful team.
Check out some of the highlights from our celebration! (more…)
Happy sunny weekend
While Germany is rather rainy these days, our GMPTEC GmbH merchandise seems to prefer sunny places!
Reach out if you need a new bag for spending the days at the beach or if you’d like to discuss your latest single-use challenges and demands.
The GMPTEC Team wishes all of you a sunny weekend!