GMPTEC Products
GMPTEC Products
GMPTEC
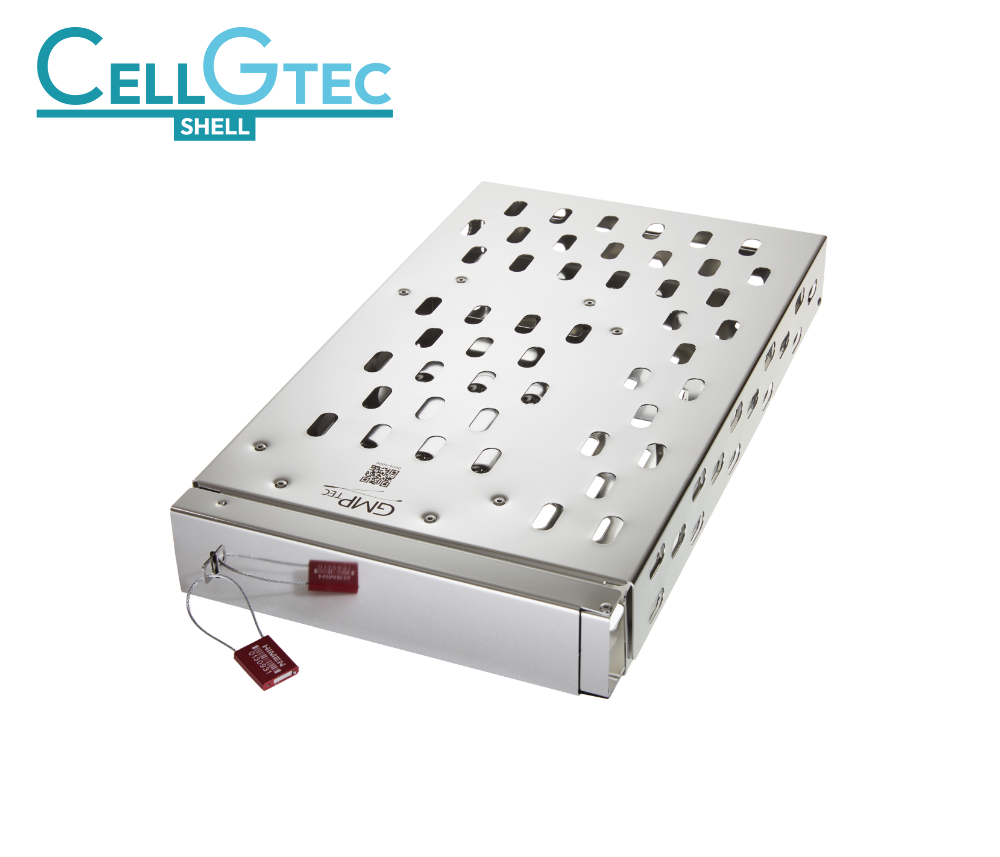
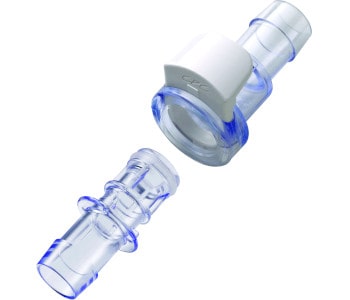
The GMPTEC product portfolio includes a huge variety of components and systems for the production of pharmaceuticals, also diagnostics, chemical products and high-quality foods. Most of the GMPTEC technologies are being used in the biotechnology and both, our single-use and multi-use components, are suitable for cGMP conform biotech-applications. GMPTECs primary goal is to cover all requirements out of one source! This does not only include a huge range of different products, but also services like consulting, assessment and troubleshooting, as well as development and construction.
In terms of customized assemblies, we will gladly take care of the whole product development process! From the first draft, to a technical drawing, to a competitive offer – completely free of charge! Our cleanroom services allow us to create another added value for your processes! We do not only deliver off-the-shelf products in big standardized packaging units: To meet your individual requirements we can clean single GMPTEC goods, channel them into the ISO class 7 cleanroom, also label or laser engrave them, repackage them individually and deliver them directly to you. Right from the start, we make your solutions and interests to our first priority!
We are always available by phone or e-mail and willing to schedule meetings on site even at short notice. The core competence of our team is their wide technical knowledge about the production technologies on the market. We do not settle for only few key products. Instead we want to offer seamless solutions, even if it should be beyond our own portfolio.
GMPTEC
The GMPTEC product portfolio includes a huge variety of components and systems for the production of pharmaceuticals, also diagnostics, chemical products and high-quality foods. Most of the GMPTEC technologies are being used in the biotechnology and both, our single-use and multi-use components, are suitable for cGMP conform biotech-applications. GMPTECs primary goal is to cover all requirements out of one source! This does not only include a huge range of different products, but also services like consulting, assessment and troubleshooting, as well as development and construction.
In terms of customized assemblies, we will gladly take care of the whole product development process! From the first draft, to a technical drawing, to a competitive offer – completely free of charge! Our cleanroom services allow us to create another added value for your processes! We do not only deliver off-the-shelf products in big standardized packaging units: To meet your individual requirements we can clean single GMPTEC goods, channel them into the ISO class 7 cleanroom, also label or laser engrave them, repackage them individually and deliver them directly to you. Right from the start, we make your solutions and interests to our first priority!
We are always available by phone or e-mail and willing to schedule meetings on site even at short notice. The core competence of our team is their wide technical knowledge about the production technologies on the market. We do not settle for only few key products. Instead we want to offer seamless solutions, even if it should be beyond our own portfolio.
NEWS
𝗪𝗲 𝗮𝗿𝗲 𝗲𝘅𝗰𝗶𝘁𝗲𝗱 𝘁𝗼 𝘄𝗲𝗹𝗰𝗼𝗺𝗲 𝗚𝗠𝗣𝗧𝗘𝗖 𝗚𝗺𝗯𝗛 𝗮𝘀 𝗮𝗻 𝗲𝘅𝗵𝗶𝗯𝗶𝘁𝗼𝗿 𝗮𝘁 𝗦𝗶𝗻𝗴𝗹𝗲-𝗨𝘀𝗲 𝗘𝘃𝗲𝗻𝘁 𝟮𝟬𝟮𝟱!
GMPTEC GmbH is your reliable partner in the biotechnology and pharmaceutical industry, with a focus on innovative single-use technologies and products. Their team of specialists and industry experts develops and produces customer-specific single-use assemblies and individual process solutions at their site in Gronau (Leine), Germany. Connect with GMPTEC GmbH and all the other participating exhibitors at Single-Use Event 2025, exchange ideas, and grow your network. Register now: https://lnkd.in/e_ze3psb View all the exhibitors of Single-Use Event 2025 here: https://lnkd.in/eZ5KuBtU
Summer, sunshine, and GMPTEC vibes!
☀️ Summer, sunshine, and GMPTEC vibes! 🍹🎉
This month, we celebrated our annual summer party – and what a day it was! Perfect weather, great food, plenty of laughter, and fantastic team spirit all around. 🌭🍦🌿
Moments like these remind us how valuable it is to connect beyond the daily routine and enjoy each other’s company in a relaxed atmosphere. A big thank you to everyone who joined – you made this day truly special!
📸 Here are a few snapshots of the fun – already looking forward to next year!
5th BIOTECH Conference 2025
Next Stop: Freiburg!
We’re excited to announce that GMPTEC will be exhibiting next week at the 5th BIOTECH Conference 2025, hosted by DECHEMA in Freiburg, Germany.
This renowned event brings together innovators, researchers, and solution providers from across the bioprocessing and biotechnology space — and we’re proud to be part of it.
🚀 We’ll be showcasing our latest Single-Use Systems for the pharmaceutical and biopharmaceutical industries – designed for maximum reliability, flexibility, and regulatory compliance.
📍 Visit our booth to learn how our solutions support the future of biotech manufacturing.
🗓️ 16th – 18th June 2025
Spontaneous Pizza Friday at GMPTEC!
Sometimes the best ideas are the unplanned ones, like this week – a pizza afternoon with the Friday afternoon crew!
With a few ingredients and plenty of team spirit, we turned the GMPTEC garden into our own little pizzeria.
A perfect way to wrap up the week and enjoy some well-deserved downtime together.
Swipe through for a few tasty impressions! (more…)
We’re live at GMP-PharmaCongress & GMP-PharmaTechnica 2025
We’re live at GMP-PharmaCongress & GMP-PharmaTechnica 2025 – and excited to showcase our innovative Single-Use portfolio at the GMPTEC GmbH booth!
As announced last week, we’re here with our dedicated team, ready to connect, discuss, and demonstrate how our solutions can take your processes to the next level.
We’re also proud to introduce our new partner for the Benelux region: Ron van Dooren from Flowmeister B.V. will now be supporting our customers in Belgium, the Netherlands, and Luxembourg, bringing his expertise and passion for innovation to the GMPTEC network.
If you’re attending PharmaTechnica – come by, meet the team, and discover what’s next in Single-Use technology!
We’re exhibiting at PharmaTechnica 2025 in Wiesbaden!
GMPTEC will be showcasing innovative Single-Use Solutions for the biopharmaceutical industry at booth B15 next week.
💡 We’re excited to be joined by our partner Flowmeister B.V., represented by Ron van Dooren, as we present cutting-edge technologies that help streamline and secure pharma production processes.
Come by, meet our team, and discover how our solutions can elevate your manufacturing operations!
📍 PharmaTechnica 2025
📆 Next week
📌 Wiesbaden | Booth B15